Wegovy® and MACE Risk Reduction
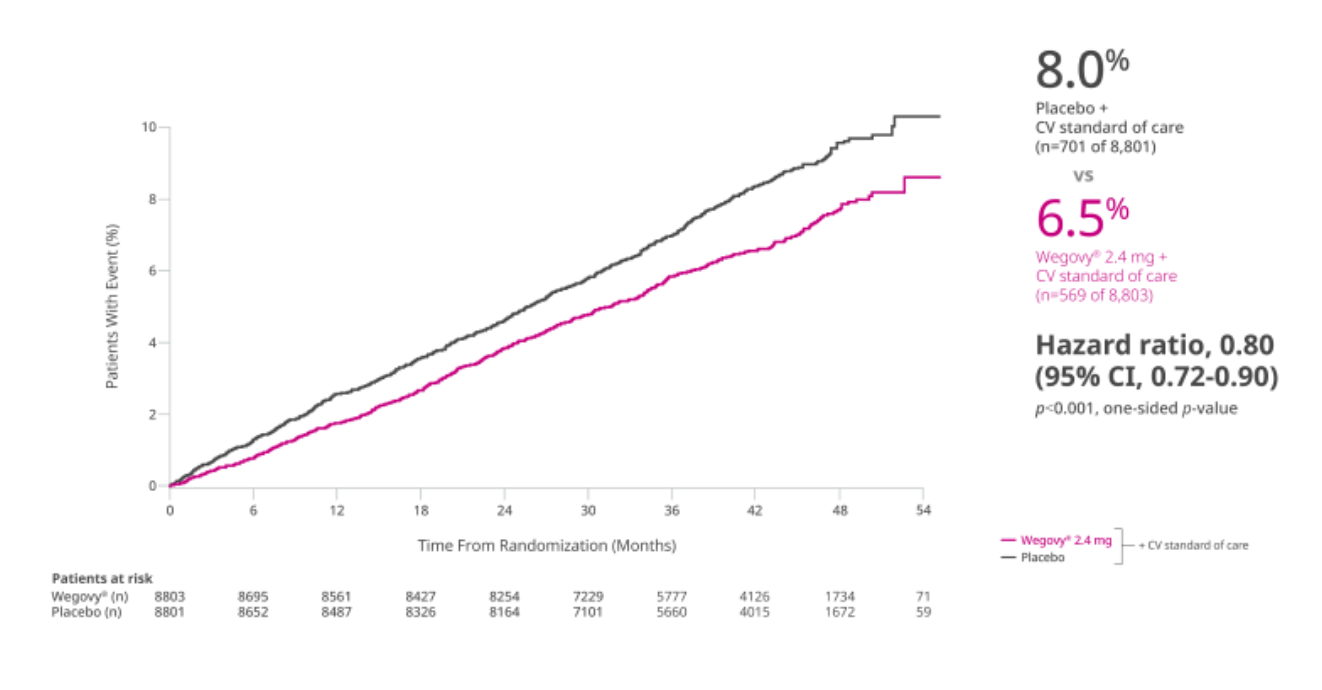
In adults with established CVD and either obesity or overweight, without diabetes, when added to CV standard of care,
Wegovy® is the first obesity treatment proven to prevent life-threatening cardiovascular events1-3
Primary composite end point: Time to first occurrence of MACE (CV death, non-fatal MI, or non-fatal stroke)1,2
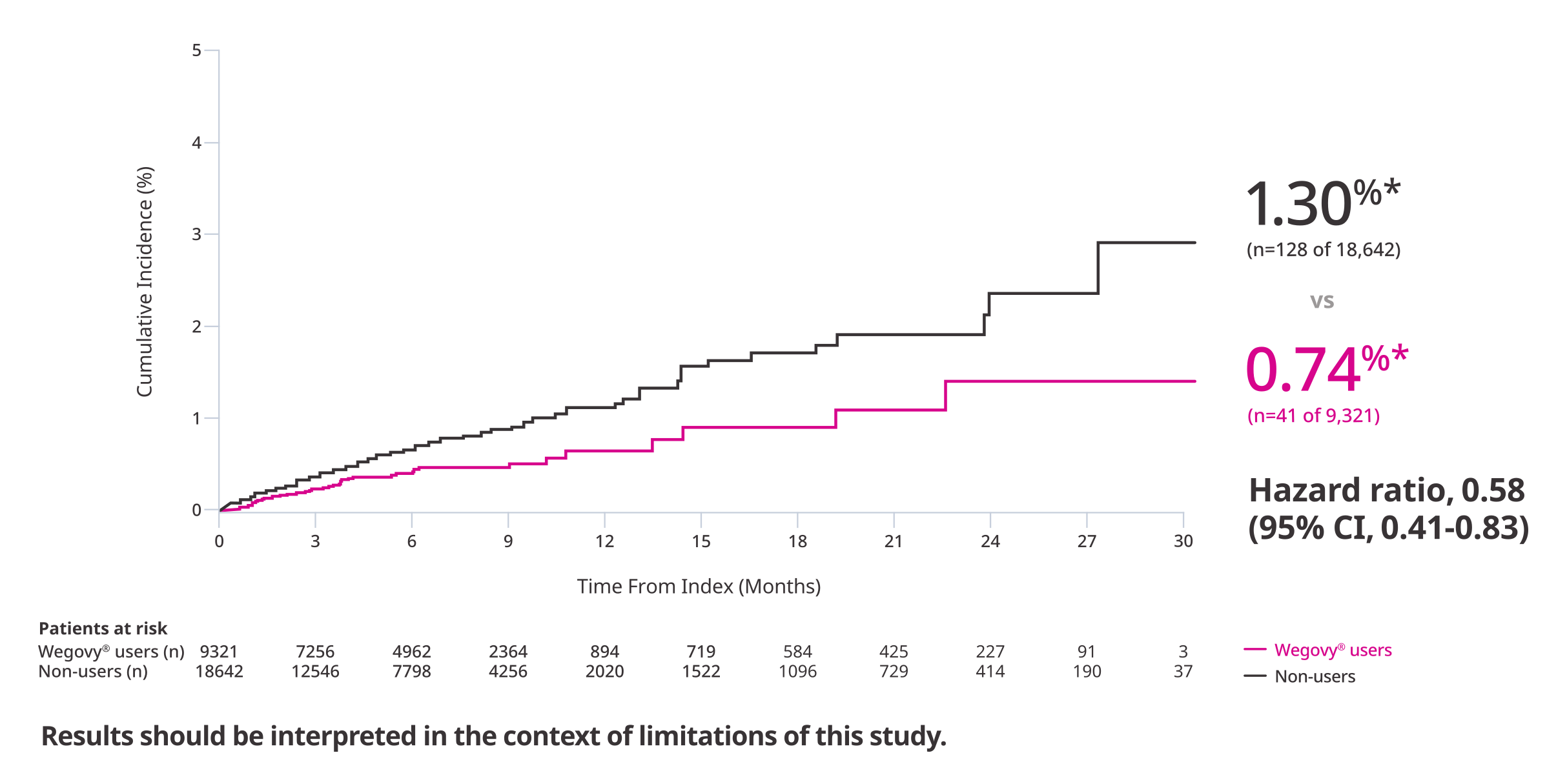
Cumulative Incidence Function: Time to first occurrence of 3-part MACE. Data from the in-trial period.
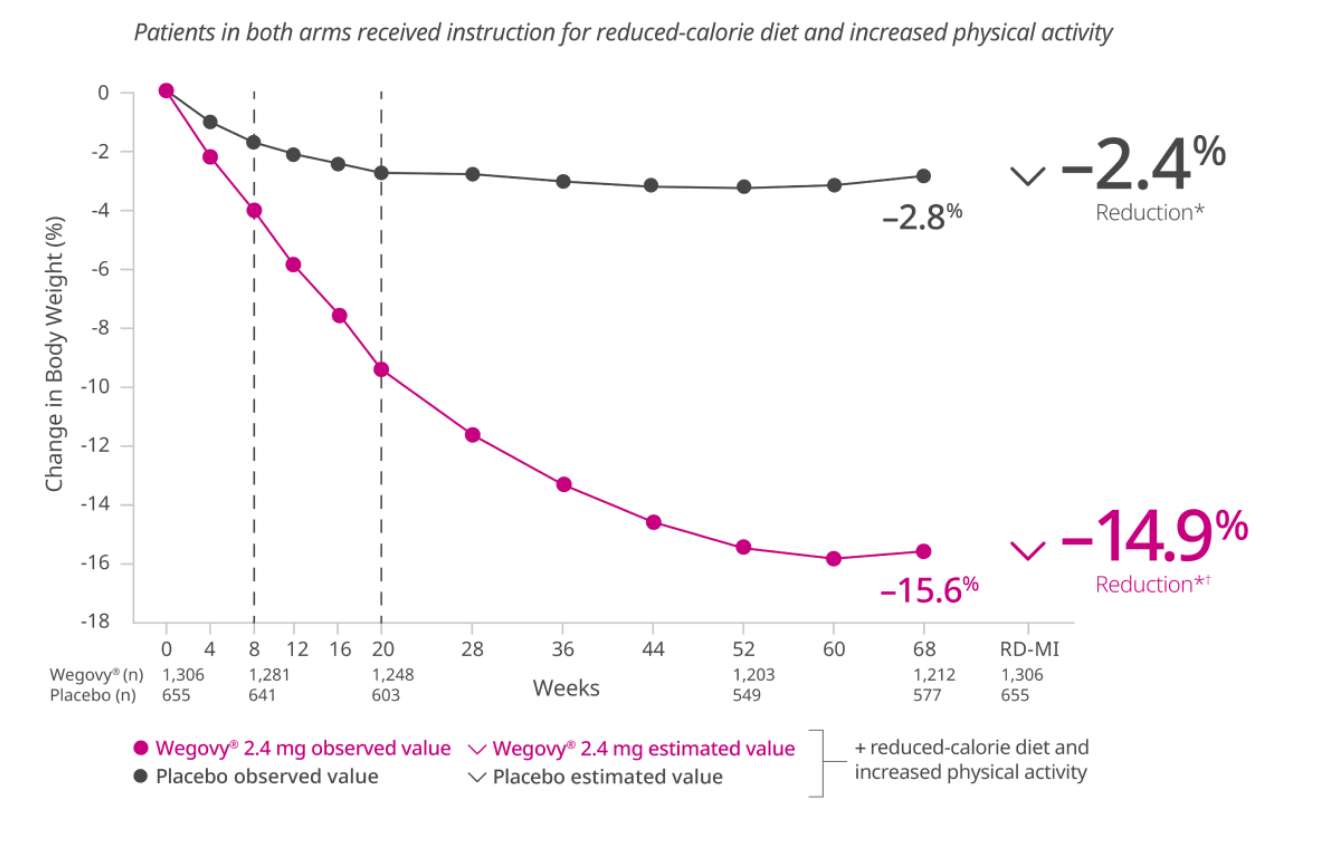
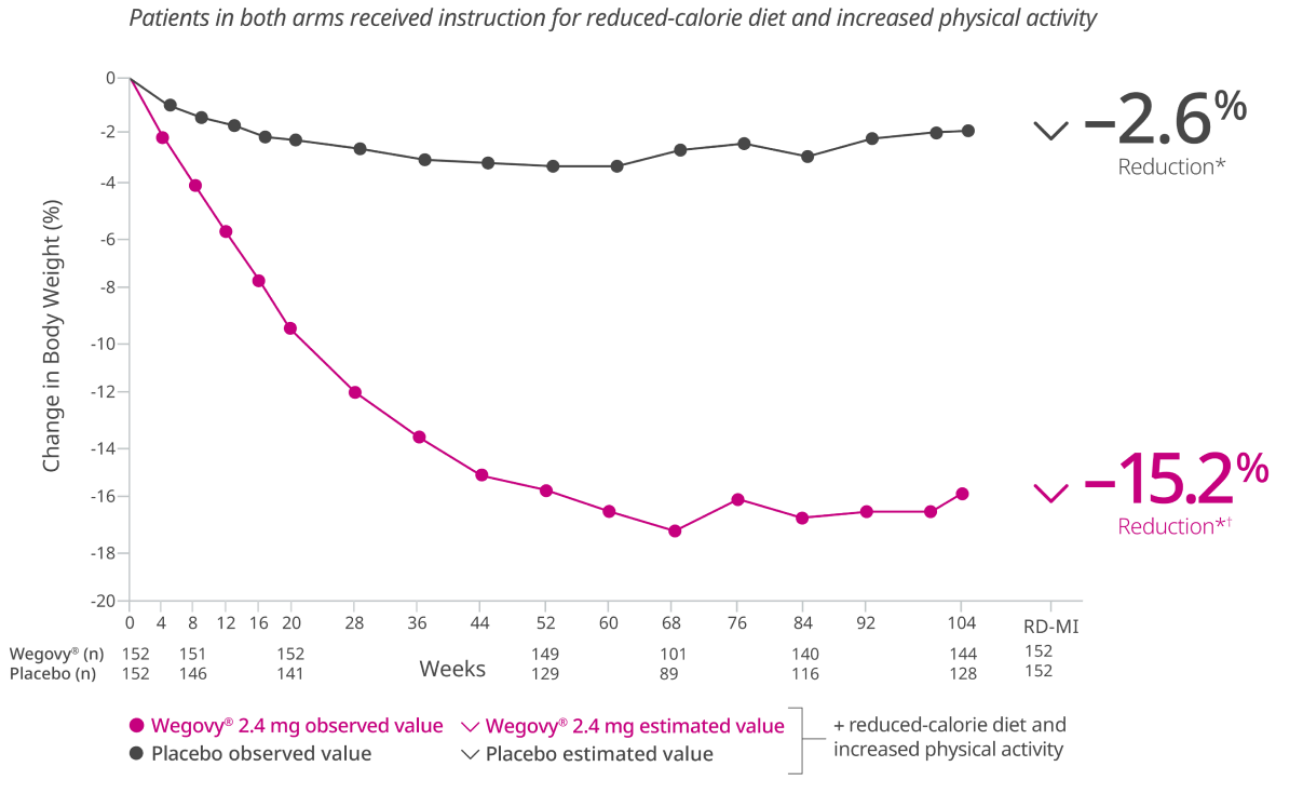
During the trial, 31% of patients in the Wegovy® arm discontinued treatment compared with 27% in the placebo arm.
SCORE real-world data: CV outcomes
When added to CV SOC1,2
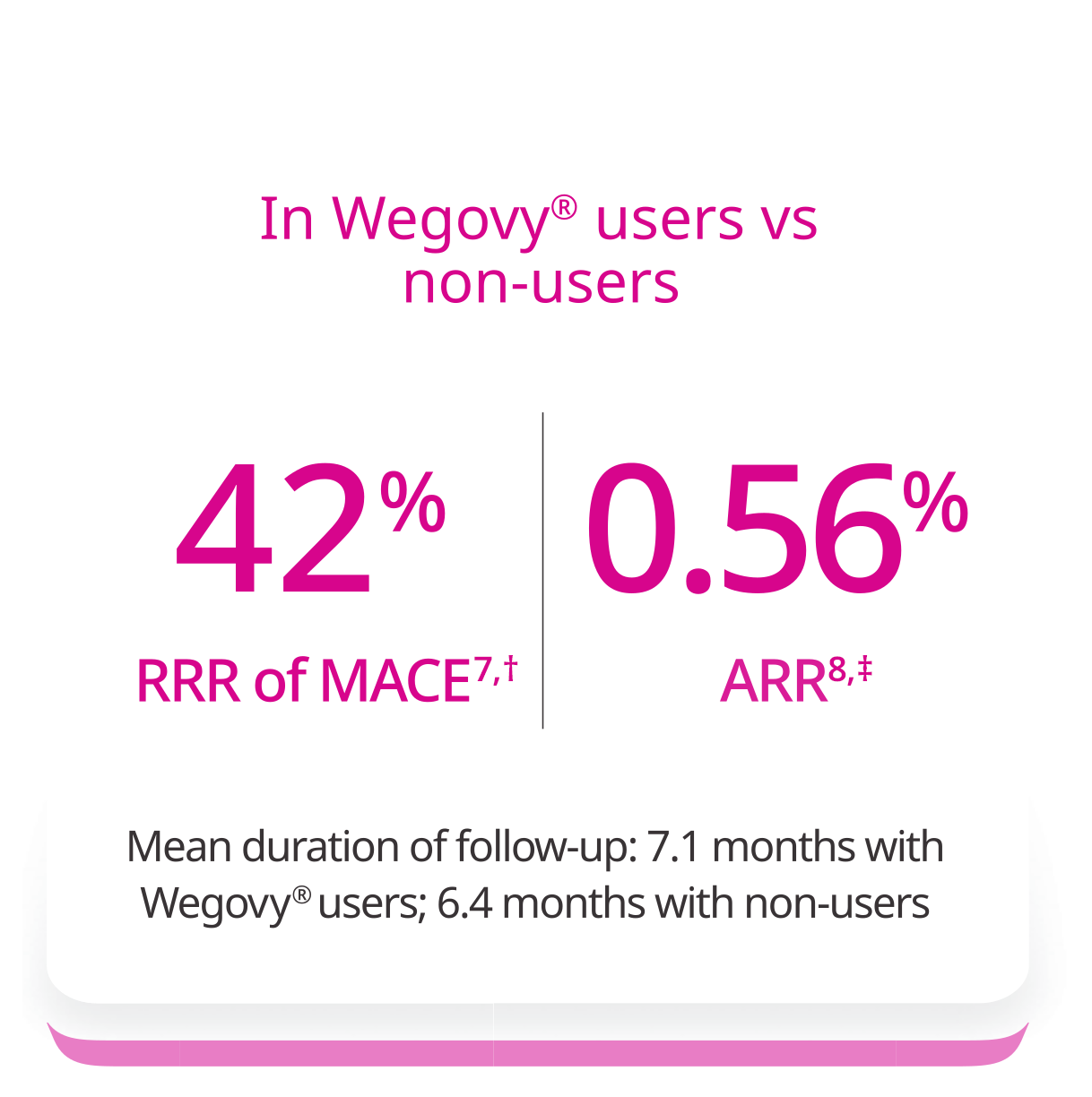
*1.5% ARR at 40 months (mean duration of follow-up).
Clinical trial
SELECT Study Design1,2
A multi-national, double-blind, placebo-controlled, event-driven CV outcomes trial of 17,604 adults with a BMI ≥27 kg/m2 and established CVD (prior MI, prior stroke, or PAD) assessing superiority of once-weekly Wegovy® 2.4 mg vs placebo (1:1 randomization, with a 16-week dose escalation) for time to first MACE. Both groups received current standard of care, including CV risk factor management and individualized healthy lifestyle counseling (including diet and physical activity); concomitant CV therapies could be adjusted at investigator discretion to ensure participants were treated according to the current standard of care for patients with established CVD. Patients with a history of type 1 or type 2 diabetes were excluded. Median duration of follow-up was 41.8 months.
Randomized clinical trials are designed to show causality4,5
Randomized clinical trials (RCTs)
- Evaluate the safety and efficacy of a treatment in specific populations under controlled conditions
- Prospective designs with prespecified, well-defined inclusion/exclusion criteria, outcomes, and end points
- Patients are randomly assigned to treatment or comparator
Limitations:
- Tightly controlled conditions and inclusion/exclusion criteria of RCTs may limit generalizability to real-world conditions or clinical populations
- Can be expensive and time-consuming
Real-world studies evaluate associations and cannot determine causality5,6
Real-world observational studies
- Use data from routine clinical practice
- Capture outcomes across broad patient populations
- Offer insight into how treatments perform outside of a controlled clinical trial setting
- Should be viewed as complementary to clinical trial data
Limitations:
- Susceptible to bias and confounding due to factors such as lack of randomization, missing or duplicative data, coding inaccuracies, and data variability reflecting routine clinical practice
In adults with established CVD and either obesity or overweight, without diabetes,
Wegovy® was associated with lower incidence of MACE in a real-world study7
Outcome: Time to first occurrence of MACE-3 (CV death, non-fatal MI, or non-fatal stroke)7
†The relative risk reduction is approximated by 1-hazard ratio.
‡ARR represents the difference in event rates per person-year in semaglutide users versus non-users.
Limitations: Despite robust PS matching and multiple sensitivity analyses, the potential for residual unmeasured confounding exists. The approval of Wegovy® in 2021 resulted in a relatively short duration of follow-up, limiting assessment of long-term outcomes. Requiring 12 months of continuous coverage before the index date may exclude patients with intermittent insurance coverage of those from underserved populations limiting generalizability.
Real-world evidence
SCORE Study Design7
A retrospective observational study using the Komodo Research Dataset (EHR linked to medical and pharmacy claims) of 27,963 US adults with a BMI ≥27 kg/m2 and established CVD (prior MI, prior stroke, or PAD) comparing the incidence of MACE-3 (CV death, non-fatal MI, or non-fatal stroke) in Wegovy® users vs non-users without diabetes. Full study period was from January 1, 2016, to December 31, 2023. Index date was defined as first Wegovy® claim or randomly selected pharmacy claim on or after June 4, 2021, for Wegovy® users and non-users, respectively. Baseline period was 12 months prior to the index date. Wegovy® users and non-users were matched using a non-parsimonious PS model to alleviate the effect of potential confounding on systematic differences in patient characteristics. Over 50 variables were matched, including age, gender, race/ethnicity, region, insurance type, index year, duration between the eligibility date and index date, BMI, smoking history, comorbidities, procedures, medication use, and health care resource utilization.
EXPLORE THE STUDY DATA
ARR, absolute risk reduction; BMI, body mass index; CI, confidence interval; CV, cardiovascular; CVD, cardiovascular disease; EHR, electronic health record; MACE, major adverse cardiovascular events; MI, myocardial infarction; PAD, peripheral arterial disease; PS, propensity score; RRR, relative risk reduction; SOC, standard of care.
References: 1. Wegovy® [package insert]. Plainsboro, NJ: Novo Nordisk Inc. 2. Lincoff AM, Brown-Frandsen K, Colhoun HM, et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med. 2023;389(24):2221-2232. 3. FDA approves first treatment to reduce risk of serious heart problems specifically in adults with obesity or overweight. FDA. Published March 8, 2024. Accessed April 23, 2025. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-reduce-risk-serious-heart-problems-specifically-adults-obesity-or 4. Zabor EC, Kaizer AM, Hobbs BP. Randomized controlled trials. Chest. 2020;158(1S):S79-S87. 5. Sheldrick RC. Randomized trials vs real-world evidence: how can both inform decision-making? JAMA. 2023;329(16):1352-1353. 6. Dang A. Real-world evidence: a primer. Pharmaceut Med. 2023;37(1):25-36. 7. Smolderen KG, Mena-Hurtado C, Zhao Z, et al. Lower risk of cardiovascular events in patients initiated on semaglutide 2.4 mg in the real-world: results from the SCORE study (Semaglutide Effects on Cardiovascular Outcomes in People with Overweight or Obesity in the Real World). Diabetes Obes Metab. 2025;27(11):6691-6704. Published online September 9, 2025. doi:10.1111/dom.70080 8. Data on file. Novo Nordisk Inc.; Plainsboro, NJ.